Rapid fFN® Test
Quantitative fetal fibronectin (fFN) testing reliably indicates if a woman is likely to give birth prematurely.1

Symptoms May Be Real, but the Risk Is Not
When a patient has symptoms of preterm labour, it is important to determine her risk of preterm birth.2
The Rapid fFN Test is the only named biomarker test recommended in the National Institute for Health and Care Excellence (NICE) clinical guidelines for the risk assessment of preterm labour & birth.3
Insight into the Risk of Early Delivery
Preterm birth risk can be assessed by labour symptoms such as uterine contractions, cervical change and premature rupture of membranes. However, these biophysical markers are not as accurate in predicting preterm birth as fetal fibronectin, a biochemical marker that can often be detected before the biophysical markers.4-6
Establish Risk
Collect definitive data to better predict the likelihood of preterm birth in just 5 steps, with results ready in 10 minutes.4
Manage Care
Admit, observe or discharge? Quantitative fFN testing detects the concentration levels of fetal fibronectin (fFN), allowing you to determine risk of preterm delivery.
Peace of Mind
Gives women at low risk of preterm labour peace of mind in knowing that they should not deliver early. For those at higher risk, you can reassure them that they are getting the right care.
Improving Patient Care is Within Your Control
For many women, the diagnosis and treatment of uterine disorders is a long, painful and frustrating journey. Our team pioneers gynaecological diagnostic and surgical solutions that give professionals greater confidence in patient diagnosis and more choice over treatment options.
The Rapid fFN Test is part of the Hologic Uterine Health Portfolio.

More Information & Less Guesswork
Fetal fibronectin (fFN) is a “glue-like” protein that helps bond the baby to the uterus. It is detectable in vaginal secretions at the beginning of pregnancy, when this bond is first forming, and then again at the end of pregnancy. If fetal fibronectin is detectable between 22 and 35+6 weeks in symptomatic women2, this could indicate the “glue” is leaking and preterm delivery is a real possibility. Between 18 and 27+6 weeks it is also possible to test asymptomatic women with a high-risk of preterm labour.2
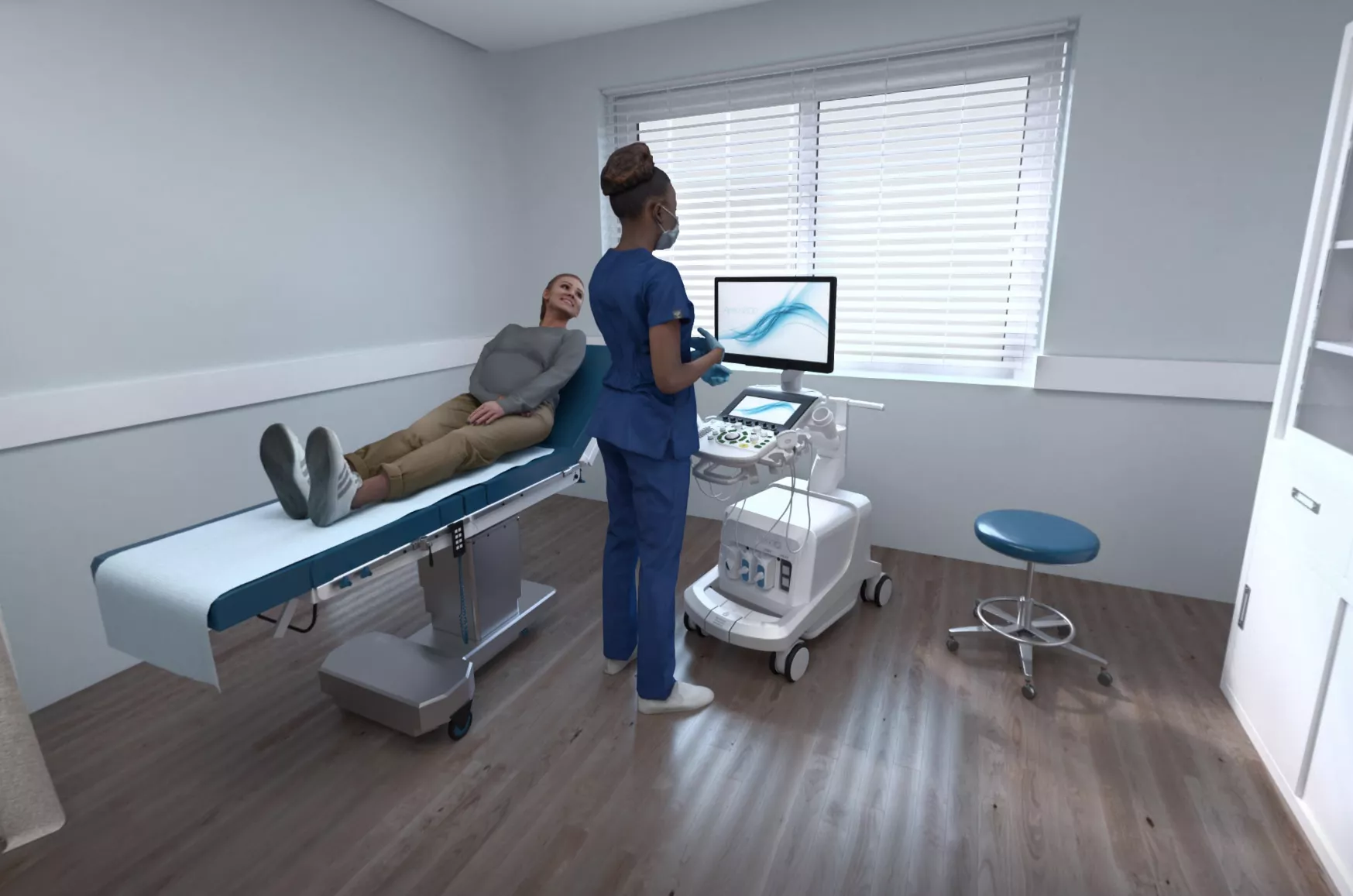
Visit Our Virtual Hospital
See how our Uterine Health products work in an outpatient and operating room (OR) setting, including a Perinatal Suite.
Quantitative Data for a More Complete Answer
Our quantitative Rapid fFN Test on the PeriLynx System gives healthcare professionals valuable information in minutes. You can use our products with the 3rd party QUiPP app*, a clinical decision-making tool.
Preterm Birth Continues to Rise Worldwide
In the UK, 8% of babies are born prematurely (approximately 60,000 births per year).8 When a patient experiences symptoms of preterm labour, you should determine if her risk of preterm birth is real or perhaps quite low. In the UK, threatened preterm labour accounts for an estimated 26% of antenatal admissions9 but more than 95% of these women don't go on to deliver within 14 days.10
Evidence. Insight. Collaboration.
Our education portal improves patient care through excellence in education, communication of clinical and scientific evidence, and partnerships with the healthcare community.
Insights
*The QUiPP app is not a Hologic product. Hologic makes no warranties, express or implied, with respect to this product. Please reach out to the QUiPP team and access the QUiPP app Toolkit for further information.
-
National Institute for Health and Care Excellence (NICE). Diagnostic consultation document. March 2018. Available at: https://www.nice.org.uk/guidance/gid-dg10017/documents/diagnostics-consultation-document.
-
AW-29099-001 Rev 002 – 10Q Cassette Product Insert
-
National Institute for Health and Care Excellence (NICE). Preterm labour and birth guidance. Published November 2015. Available at: https://www.nice.org.uk/guidance/ng25/chapter/Recommendations. Section 1.7.5.
-
Wadhwa PD, Culhane JF, Rauh V, et al. Stress, infection and preterm birth: a biobehavioural perspective. Paediatric Perinat Epidemiol. 2001;15(suppl 2):17-29
-
Lockwood CJ, Kuczynski E. Risk stratification and pathological mechanisms in preterm delivery. Paediatric Perinat Epidemiol. 2001;15(suppl 2):78-89
-
Goldenberg RL, Iams JD, Mercer BM, et al. The Preterm Prediction Study: The value of new vs standard risk factors in prediction early and all spontaneous preterm births. Am J Public Health. 1998;88:233-38
-
AW-24885-001 Rev 002 Perilynx System User Manual
-
Guys’ and St. Thomas’ NHS Foundation Trust. 2012 news items. Simple test will help predict and prevent premature births. November 2012. Available at: Guys and St Thomas – Predict Preterm Labour Last accessed on 30 September 2022.
-
Parisaei M, Currie J, O’Gorman N, et al. Implementation of fetal fibronectin testing: admissions, maternal interventions and costs at 1 year. J Obstet Gynaecol 2016; 36(7): 888-892
-
Peaceman AM, Andrews WW, Thorp JM, et al. Fetal fibronectin as a predictor of preterm birth in patients with symptoms: a multicenter trial. Am J Obstet Gynecol 1997;177:13-8.
Safety Data Sheets
Package Inserts
Frequently Asked Questions
Fetal fibronectin (fFN) is a “glue-like” protein that helps bond the baby to the uterus.
Fetal fibronectin is detectable in vaginal secretions in the very beginning of pregnancy, when this bond is first forming, and then again at the end of pregnancy, when the body is getting ready to deliver the baby. If fetal fibronectin is detectable in vaginal discharge between 22 and 35+6 weeks in symptomatic women1, this could indicate the “glue” is leaking prematurely and preterm delivery is a real possibility.
It is also possible to test asymptomatic women at high-risk for preterm labour between 18 and 27+6 weeks.1
In the majority of maternity hospitals (over 130) in the UK.2
Patients presenting with signs or symptoms of preterm labour are eligible to receive a Rapid fFN Test.1 These may include:3
- Either a slow trickle or a gush of clear or pinkish fluid from the vagina or any increase in vaginal discharge
- Backache
- Cramps like strong period pains
- A frequent need to urinate
- A feeling of pressure in the pelvis
- Nausea, vomiting or diarrhoea.
There are studies to support the use of fFN testing in twins.
Asymptomatic patients at high risk for preterm birth can also receive a Rapid fFN Test as per the Saving Babies’ Lives Care Bundle, Version 2, Element 5.6
Rapid fFN Testing is CE Marked and licensed for use between 22 and 35+6 weeks in symptomatic women and between 18 and 27+6 weeks in asymptomatic high-risk women.1
To determine a woman’s risk of preterm delivery in the next 7-14 days.1
The test result can also give an indication of the woman’s risk of delivery up to 37 weeks utilising the QUiPP App* (a third party clinical decision-making tool that may help predict spontaneous preterm birth).5
Results can be useful when trying to determine whether to admit or transfer her and when to administer interventions such as antenatal corticosteroids, tocolysis and Magnesium Sulphate.
The specimen must be collected prior to other vaginal exams, without gels or lubricants.
- During speculum exam, lightly rotate swab across posterior fornix of vagina for 10 seconds to absorb cervicovaginal secretions.
- Remove swab and immerse polyester tip in buffer; break shaft at score even with top of tube.
- Align the shaft with hole inside the tube cap and push down tightly over shaft, sealing tube.
It is important to note that the Specimen Collection Kit for the Rapid fFN Test is at no additional cost.
Therefore, if you are unsure prior to vaginal examination or additional tests whether any of the following apply, you can take your specimen and subsequently discard it if not required.
Contraindications:
- Cervical dilation more than 3cm
- Rupture of amniotic membranes
- Cervical cerclage
- Placenta previa
- Moderate or gross vaginal bleeding*
Precautions:
- Specimen contaminated with blood
- Sexual intercourse within the previous 24 hours
* Moderate or gross bleeding is an independent risk factor for preterm delivery and may be associated with other severe obstetrical or medical problems.
Presence of blood or semen in a Rapid fFN Test sample can sometimes lead to a falsely elevated result, however results under the specified threshold for treatment at your facility can still be considered valid in either of these situations.
Yes, semen in a Rapid fFN Test sample can sometimes lead to a falsely elevated result, however results under the specified threshold for treatment at your facility can still be considered valid in this situation.
Yes, blood in a Rapid fFN Test sample can sometimes lead to a falsely elevated result, however results under the specified threshold for treatment at your facility can still be considered valid in this situation.
It is important to note however that moderate or gross vaginal bleeding is both a contraindication and is an independent risk factor for preterm delivery and may be associated with other severe obstetrical or medical problems.
Please refer to your hospital’s individual protocol regarding how to interpret your Rapid fFN Test result as this may vary dependent on whether you are using qualitative fFN, quantitative fFN or the QUiPP App* (a third party clinical decision-making tool that may help predict spontaneous preterm birth).5
If you are utilising quantitative fFN, the vast majority of patient (approximately 90%) will receive a low result allowing you to focus on the 10% that really are at the highest risk of preterm delivery.1,7
Once the specimen has been collected, it takes approximately 10 minutes for the PeriLynx Analyser to produce a quantitative fFN test result.4
The specimen can be stored at room temperature for up to 8 hours before testing or kept refrigerated for up to 3 days before testing.4
The Rapid fFN Test is on the NHS Supply Chain New Pathology & POCT Framework in the UK. Please feel free to contact us to discuss pricing further. Please note that the Specimen Collection Kits are at no additional cost when purchasing our 10Q Cassettes. This is to allow you to take a patient sample prior to vaginal examination and additional tests then discard if not required.
-
AW-29099-001 Rev 002 - 10Q Cassette Product Insert
-
Hologic Ltd. Data On File
-
Tommy’s Signs of Premature Labour
-
SS-00976-EUR-EN Rev 001 – Rapid fFN PeriLynx System Quick Reference Guide
-
QUiPP App Clinical Decision Making Tool – (last accessed 27th August 2020)
-
Saving Babies Lives (Version Two)
-
Abbott DS, Radford SK, Seed PT, Tribe RM, Shennan AH. Evaluation of a quantitative fetal fibronectin test for spontaneous preterm birth in symptomatic women. Am J Obstet Gynecol. 2013;208(2):122.e1-122.e1226. doi:10.1016/j.ajog.2012.10.890
Related Products
2797
Hologic BV, Da Vincilaan 5, 1930 Zaventem, Belgium.
Notified Body number wherever applicable
EC Representative Information wherever applicable