Breast Biopsy Solution
A comprehensive portfolio of clinically proven biopsy solutions from the world leader in breast health.

The Most Recommended Brand in Women’s Imaging & Breast Biopsy¹
It is our mission to transform breast care with compassionate and effective solutions designed to maximise value, while minimising the negative impacts of interventional procedures. The best technologies should ease patient anxiety and help clinicians focus on what’s most important, providing the best possible care. That’s why our curated portfolio of interventional products is supported by clinically proven outcomes and optimised for the future of patient care.
Optimise Workflows
Solutions designed to save time by combining and eliminating steps in the biopsy process.
Quick setup times to keep procedures on track and on time.
Create a Better Patient Experience
Fast, efficient procedures that minimise time spent under compression.
Integrated pain management to address discomfort.
Deliver the Best Outcomes Possible
Confidently approach challenging lesions with clinically proven solutions to acquire the tissue you need for diagnosis.
Give more women an alternative to open surgical biopsy so they can get back to their lives, sooner.
Decades of Continuous Innovation
13 min
time saved on average per biopsy procedure with Brevera® Breast Biopsy System2,3
360 ̊
breast access with the world first and only dedicated Affirm® Prone Breast Biopsy System
3x exam
options with the MRI, ultrasound or stereotactic compatible ATEC® Breast Biopsy System
Under 30s
to remove cores using the Eviva® Breast Biopsy System, with 4.5s cycle time5
A Comprehensive Range of Clinically Proven Solutions
We bring imaging and intervention together to offer a robust range of products within our Breast Biopsy Solution. We will continue to work with physicians to discover and deliver more compassionate and effective solutions for women. There are different levels of investments available and we support you in finding the right solution tailored to your needs.

Brevera® Breast Biopsy System
Reducing time under compression for your patients during a breast biopsy procedure is essential to deliver an improved patient experience. The Brevera Breast Biopsy system in combination with CorLumina® Imaging Technology streamlines the entire breast biopsy process from start to finish - with real-time imaging for instant verification and automated post-biopsy specimen handling.2-4

Eviva® Breast Biopsy System
The Eviva Breast Biopsy System was designed with the flagship technology of the Hologic ATEC Breast Biopsy Device, with additional features for optimal use during stereotactic or 3D biopsy procedures. Breast biopsies using Eviva provides clinicians with the highest quality core samples.6,7

ATEC® Breast Biopsy System
The ATEC® Sapphire vacuum-assisted breast biopsy console is a simple, all-in-one platform for any modality: ultrasound, MRI, stereotactic 2D and 3D image guided biopsy. Designed for radiologists and surgeons who need a simple solution with maximum utility, and considers compassionate care and confident clinical results.

Breast Biopsy Site Markers
The Hologic range of biopsy site markers come in multiple shapes, gauges and lengths. The markers are compatible for use with x-ray, ultrasound and MRI guided biopsies. Distinct shapes with options for great long-term visibility, our markers are designed so you can identify sites with confidence. An easy to use deployment device is included.
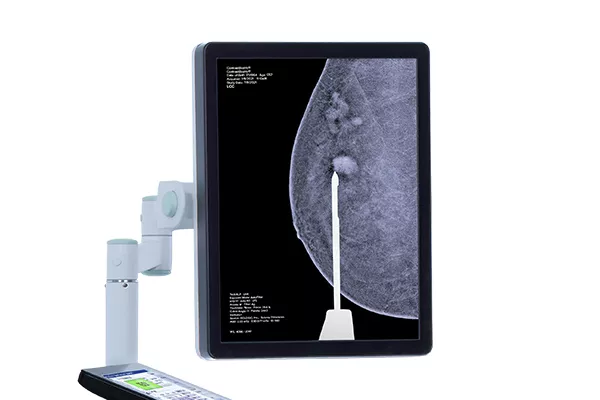
Affirm® Contrast Biopsy Software
Target and acquire breast tissue identified with CEM and simplify the breast biopsy process, even for the most challenging lesions.8 Deliver a more efficient breast biopsy procedure with the combination of I-View Contrast-Enhanced Imaging, and the Affirm Upright Breast Biopsy Guidance System.8,9

Affirm® Prone Breast Biopsy System
Hologic introduces the world’s first and only dedicated prone biopsy system capable of both stereotactic and tomosynthesis- guided procedures. The system delivers faster, more comfortable procedures by increasing automation, providing superior imaging and giving 360° access to the breast, creating a better experience for patients, technologists and radiologists.6
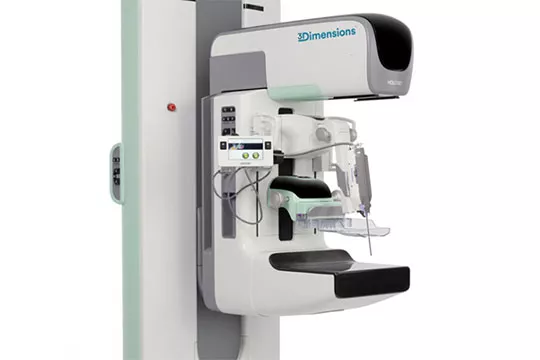
Affirm® Upright Breast Biopsy Guidance System
This simple add-on to any Hologic 3D Mammography™ capable system enables you to quickly perform 2D or 3D™ breast biopsies using the same imaging equipment as for mammography exams. Plus, with the lateral arm upright biopsy accessory, you have even more flexibility to access challenging lesion locations.
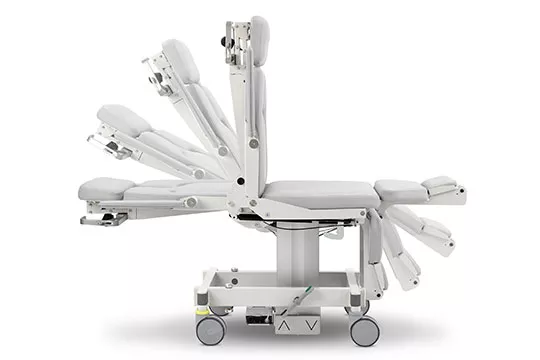
Akrus™ Mammography Positioning Chair
The Akrus Mammography and Biopsy Chair is versatile and ergonomically designed offering flexibility with patient comfort in mind. It allows a variety of positioning options including upright and decubitus in all mammography projections including magnification views. A small footprint providing technologists ease of use and maximum comfort for the patient during a biopsy.
Insights
Visit Our Virtual Hospital
Browse our portfolio of Breast Health solutions in 3D. See how you can unlock the advantage of time across the entire Breast Continuum of Care.

- Hologic Data on File, MISC-06068, Rev 001. Results from Eviva Audit, Kadence International, July 2015.
- Hologic Data on File: MISC-07678-EUR-EN, Rev 001.
- Hologic Data on File: MISC-07662-EUR-EN, Rev 001. Annotation: Inspired Insight online survey, April 2019 with 49 Brevera® users.
- Hologic Data on File: VAR-05326, Rev 002.
- Hologic Data on File: VER-03567, Rev 001.
- Compared to MultiCare® Platinum System.
- Hologic Data On File: MISC-06068 Rev 001. Eviva Audit, 155 respondents comprised of radiologists, RAD Admins, Directors of Radiology, and Breast Surgeons. Identifying Optimal Claims for the Eviva Breast Biopsy Device, July 2015. Kadence International report released April, 2020.
- Schrading S, Martine D, Dirrichs T, et al. “Digital Breast Tomosynthesis-guided Vacuum-assisted Breast Biopsy: initial Experiences and Comparison with Prone Sterotactic Vacuum-assisted Biopsy.” Radiology. 2015 274:3, 654-662 E-pub 2014 Nov 12.
- PLN-10394_001_01 – Affirm Contrast Biopsy Study.pdf.
Related Portfolio & Solutions
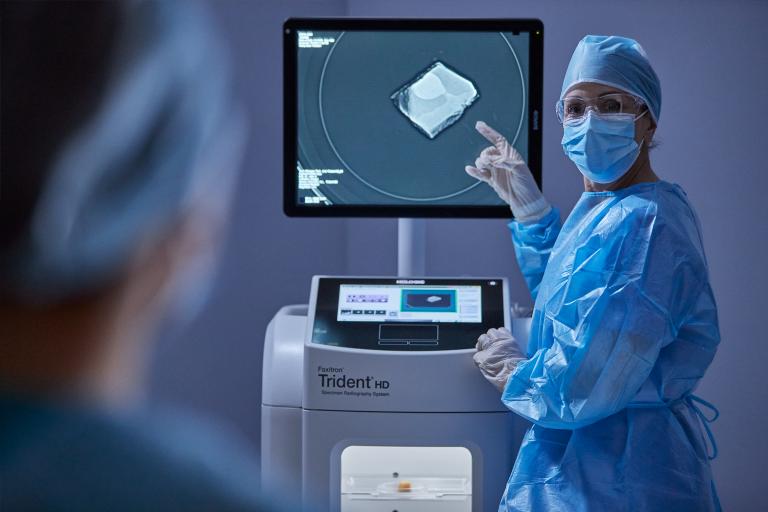
Breast Health Continuum of Care
Time is precious when it comes to effective detection, diagnosis and treatment of breast cancer. We strive to save you time at every step along the Breast Health Continuum of Care ensuring more women have more time in better health.
2797
0482
Hologic BV, Da Vincilaan 5, 1930 Zaventem, Belgium.
Notified Body number wherever applicable
EC Representative Information wherever applicable



















